Fuel is a substance which, when burnt, i.e. on coming in contact and reacting with oxygen or air, produces heat. Thus, the substances classified as fuel must necessarily contain one or several of the combustible elements : carbon, hydrogen, sulfur, etc. In the process of combustion, the chemical energy of fuel is converted into heat energy. To utilize the energy of fuel in most usable form, it is required to transform the fuel from its one state to another, i.e. from solid to liquid or gaseous state, liquid to gaseous state, or from its chemical energy to some other form of energy via single or many stages. In this way, the energy of fuels can be utilized more effectively and efficiently for various purposes.
Fuels may broadly be classified in two ways, i.e.
(a) according to the physical state in which they exist in nature – solid, liquid and gaseous, and
(b) according to the mode of their procurement – natural and manufactured.
None of these classifications, however, gives an idea of the qualitative or intensive value of the fuels, i.e. their power of developing the thermal intensity or calorimetric temperature under the normal condition of use, i.e. combustion of fuels
in mixture with atmospheric air in stoichiometric proportion.. A brief description of natural and manufactured fuels is given in the table 1. below
Table.1: Natural and Manufactured Fuels
SOLID FUELS AND THEIR CHARACTERISTICS
Solid fuels are mainly classified into two categories, i.e. natural fuels, such as wood, coal, etc. and manufactured fuels, such as charcoal, coke, briquettes, etc. (Table 1.). The various advantages and disadvantages of solid fuels are given below :
(a) They are easy to transport.
(b) They are convenient to store without any risk of spontaneous explosion.
(c) Their cost of production is low.
(d) They posses moderate ignition temperature.
Disadvantages
(c) Their cost of production is low.
(d) They posses moderate ignition temperature.
Disadvantages
(a) Their ash content is high.
(b) Their large proportion of heat is wasted.
(c) They burn with clinker formation.
(d) Their combustion operation cannot be controlled easily.
(e) Their cost of handling is high.
Woods and their Characteristics
The most commonly used and easily obtainable solid fuel is wood. It is the oldest type of fuel which man had used for centuries after the discovery of the fire itself. In India, wood is used in almost every village, as well as in small towns and cities. In some parts of country such as Kashmir and Mysore, wood is used for industrial purposes as well. Wood is vegetable tissue of trees and bushes. It consists of mainly cellular tissue and lignin and lesser parts of fat and tar, as well as sugar.
Ash
The ash content of wood is negligible. The ash consists of mineral matter that is found in the wood itself, with an admixture of some impurities which accrue during transportation, etc. The mineral matter is distributed in the tree rather irregularly. The ash consists of mainly potassium carbonate with varying degrees of calcium, magnesium and sodium carbonate, as well as minute quantities of iron oxides, alumina and silica. Pure ash is white in color.
Moisture
A freshly felled tree contains anything from 40% to 60% of hygroscopic moisture depending upon the species of the tree as well as the seasons of the year. On exposure to atmospheric air, the moisture dries up and reduces to 15-20% in about 18 months. On the exposure for a longer period, no appreciable change had been observed.
Characteristics of Flame
The nature of the flame depends on the tar content of wood. Pine and birch contain more tar and hence burn with a thick and bright flame, while aspen and alder burn with a dim, transparent flame. The length of the flame also depends on the tar content.
Combustion Characteristics
The lighter the wood, the more intensely it burns with a long flame. This is because air penetrates easily throughout the whole piece during combustion. If the wood is heavy, i.e. hard, the penetration of air is rendered difficult and a concentrated flame results with the development of more heat at the point of burning.
Ignition Temperature
Wood ignites very easily. That is why it is used for lighting other fuels.
Coal
Coal, a naturally occurring combustible solid, is one of the world's most important and abundant energy sources. From its introduction 4,000 years ago as a fuel for heating and cooking, to its nineteenth- and twentieth-century use in generating electricity and as a chemical feed-stock , coal, along with oil and natural gas, has remained an important source of energy. The United States alone has 1.7 trillion short tons of identified coal resources (natural deposits) and enough recoverable reserves (coal that can be developed for use) to meet its energy needs until the year 2225. Its demonstrated reserves include 274 billion short tons that existing technology can recover, representing 25 percent of the world's 1.08 trillion short tons of recoverable coal, and 508 billion short tons of coal that existing technology can potentially mine economically. Its recoverable reserves contain more than twice the energy of the Middle East's proven oil reserves. About 100 countries have recoverable reserves; 12 countries—among them Canada, the People's Republic of China, Russia, Poland, Australia, Great Britain, South Africa, Germany, India, Brazil, and Colombia possess the largest reserves.
Origin, Composition, and Structure of Coal
Geologists believe that underground coal deposits formed about 250–300 million years ago, when much of Earth was swamp covered with thick forest and plant growth. As the plants and trees died, they sank under Earth's wet surface, where insufficient oxygen slowed their decay and led to the formation of peat. New forests and plant life replaced the dead vegetation, and when the new forests and plants died, they also sank into the swampy ground. With the passage of time and accompanying heat buildup, underground layers of dead vegetation began to accumulate, becoming tightly packed and compressed, and gave rise to different kinds of coal, each with a different carbon concentration: anthracite, bituminous coal, sub-bituminous coal, and lignite.
Types of coal
As geological processes apply pressure to dead bio-tic matter over time, under suitable conditions it is transformed successively into
Peat, considered to be a precursor of coal. It has industrial importance as a fuel in some countries, for example, Ireland and Finland.
Lignite, also referred to as brown coal, is the lowest rank of coal and used almost exclusively as fuel for electric power generation. Jet is a compact form of lignite that is sometimes polished and has been used as an ornamental stone since the Iron Age.
Sub-bituminous coal, whose properties range from those of lignite to those of bituminous coal and are used primarily as fuel for steam-electric power generation. Additionally, it is an important source of light aromatic hydrocarbons for the chemical synthesis industry.
Bituminous coal, a dense mineral, black but sometimes dark brown, often with well-defined bands of bright and dull material, used primarily as fuel in steam-electric power generation, with substantial quantities also used for heat and power applications in manufacturing and to make coke.
Anthracite, the highest rank; a harder, glossy, black coal used primarily for residential and commercial space heating. It may be divided further into metamorphically altered bituminous coal and petrified oil, as from the deposits in Pennsylvania.
Graphite, technically the highest rank, but difficult to ignite and is not so commonly used as fuel: it is mostly used in pencils and, when powdered, as a lubricant.
Coal is classified into four main types, or ranks (lignite, sub-bituminous, bituminous, anthracite), depending on the amounts and types of carbon it contains and on the amount of heat energy it can produce. The rank of a deposit of coal depends on the pressure and heat acting on the plant debris as it sank deeper and deeper over millions of years. For the most part, the higher ranks of coal contain more heat-producing energy.
- Peat is an organic material that forms in the waterlogged, sterile, acidic conditions of bogs and fens. These conditions favour the growth of mosses, especially sphagnum. As plants die, they do not decompose. Instead, the organic matter is laid down, and slowly accumulates as peat because of the lack of oxygen in the bog. A little over 3% of the earth's land surface is covered in peat.
- Lignite is also known as brown coal and is an intermediate stage between peat and coal. Lignite was formed about 50 million years ago. It is the lowest rank of coal with the lowest energy content. Lignite coal deposits tend to be relatively young coal deposits that were not subjected to extreme heat or pressure. Lignite is crumbly and has high moisture content. Lignite is mainly burned at power plants to generate electricity.
- Sub-bituminous coal has a higher heating value than lignite. Sub-bituminous coal typically contains 35-45 percent carbon, compared to 25-35 percent for lignite. Most sub-bituminous coal in the U.S. is at least 100 million years old. About 44 percent of the coal produced in the United States is sub bituminous.
- Bituminous coal contains 45-86 percent carbon, and has two to three times the heating value of lignite. Bituminous coal was formed under high heat and pressure. Bituminous coal in the United States is between 100 to 300 million years old. It is the most abundant rank of coal found in the United States, accounting for about half of U.S. coal production. Bituminous coal is used to generate electricity and is an important fuel and raw material for the steel and iron industries.
- Anthracite contains 86-97 percent carbon, and has a heating value slightly lower than bituminous coal.
Because of its origin in ancient living matter, coal, like oil and gas, is known as a fossil fuel. It occurs in seams or veins in sedimentary rocks; formations vary in thickness, with those in underground mines 0.7–2.4 meters (2.5–8 feet) thick and those in surface mines, as in the western United States, sometimes 30.5 meters (100 feet) thick.
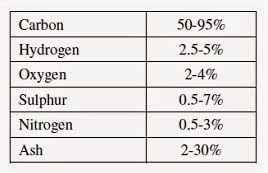
Coal contains significant carbon, and smaller percentages of the elements hydrogen, oxygen, nitrogen, and sulfur. Inorganic compounds such as aluminum and silicon oxides constitute the ash. Distillation produces tar, water, and gases. Hydrogen is the chief component of the gases liberated, although ammonia, carbon monoxide and dioxide gases, benzene and other hydrocarbon vapors are present. (The composition of a bituminous coal by percentage is roughly: carbon [C], 75–90; hydrogen [H], 4.5–5.5; nitrogen [N], 1–1.5; sulfur [S], 1–2; oxygen[O], 5–20; ash, 2–10; and moisture, 1–10.)
It is commonly adopted view that coal is a mineral substance of vegetable origin. The large deposits of coal in India are in Bengal, Bihar and Madhya Pradesh. Most of the Indian coal is of low grade variety and coal washing to obtain low ash metallurgical coal is unavoidable. Over 30% of coal output is consumed by railways, another similar proportion is used by industry including iron and steel works. This leaves barely 40% of coal mined for use of the power supply undertakings.
Analysis of Coal
To ascertain the commercial value of coal certain tests regarding its burning properties are performed before it is commercially marketed. Two commonly used tests are : Proximate analysis and Ultimate analysis of coal. Calorific value of coal is defined as the quantity of heat given out by burning one unit weight of coal in a calorimeter.
Proximate Analysis of Coal
This analysis of coal gives good indication about heating and burning properties of coal. The test gives the composition of coal in respect of moisture, volatile matter, ash and fixed carbon. The moisture test is performed by heating 1 gm of coal sample at 104 C to 110 C for 1 hour in an oven and finding the loss in weight. The volatile matter is determined by heating 1 gm of coal sample in a covered crucible at 950 C for 7 minutes and determining loss in weight, from which the moisture content as found from moisture test is deducted. Ash content is found by completely burning the sample of coal in a muffle furnace at 700 C to 750 C and weighing the residue. The percentage of fixed carbon is determined by difference when moisture, volatile matter and ash have been accounted for.
The importance of volatile matter in coal is due to the fact that it largely governs the combustion which in turn governs the design of grate and combustion's space used. High volatile matter is desirable in gas making, while low volatile matter for manufacturing of metallurgical coke.
Ultimate Analysis of Coal
This analysis of coal is more precise way to find the chemical composition of coal with respect to the elements like carbon, hydrogen, oxygen, nitrogen, sulfur and ash. The content of carbon and hydrogen combines with oxygen to form carbon dioxide and water by way of combustion. The chemical analysis of coal alone is not enough to predict the suitability of coal for purpose of heating. However, the chemical composition is very useful in combustion calculations and in finding the composition of flue gases. For most purposes the proximate analysis of coal is quite sufficient. The broad range in which the constituents of coal vary by weight as determined by ultimate analysis are given below :
Very dirty coals, those containing large amounts of incombustible material, could only qualify for substantial cleaning if the final selling price clearly justified it. For markets, coal cleaning would improve market potential of the coal and reduce transportation costs. Quality and cost of treated coals are highly dependent on the specific coal and the market for which it is aimed.
Coal preparation includes blending and homogenization, size reduction, grading, screening and handling and, perhaps most importantly, beneficiation or cleaning. Selection of methods and corresponding degree of beneficiation required, significantly governs the cost of coal preparation, which is greatly determined by the market suitability of the product. Therefore, there are different levels of cleaning to which a coal may be economically subjected, according to its intended utilization. Very dirty coals (those containing large amount of incombustible material), could only qualify for substantial cleaning if the final selling price justifies it.
The demand for coal preparation will grow along with increases in production for various reasons, some of which are mentioned as follows:
Coal cleaning is a process by which impurities such as sulfur, ash, and rock are removed from coal to upgrade its value. Coal cleaning processes are categorized as either physical cleaning or chemical cleaning. Physical coal cleaning processes, the mechanical separation of coal from its contaminants using differences in density, are by far the major processes in use today. Chemical coal cleaning processes are currently being developed, but their performance and cost are undetermined at this time. Therefore, chemical processes are not included in this discussion. In the initial preparation phase of coal cleaning, the raw coal is unloaded, stored, conveyed, crushed, and classified by screening into coarse and fine coal fractions. The size fractions are then conveyed to their respective cleaning processes.
The majority of coal cleaning processes use upward currents or pulses of a fluid such as water to fluidize a bed of crushed coal and impurities. The lighter coal particles rise and are removed from the top of the bed. The heavier impurities are removed from the bottom. Coal cleaned in the wet processes then must be dried in the final preparation processes.
Final preparation processes are used to remove moisture from coal, thereby reducing weight and raising the heating value. The first processing step is dewatering, in which a major portion of the water is removed by the use of screens, thickeners, and cyclones. The second step is normally thermal drying, achieved by any one of three dryer types: fluidized bed, flash, and multi-louvered. In the fluidized bed dryer, the coal is suspended and dried above a perforated plate by rising hot gases. In the flash dryer, coal is fed into a stream of hot gases for instantaneous drying. The dried coal and wet gases are both drawn up a drying column and into a cyclone for separation. In the multi-louvered dryer, hot gases are passed through a falling curtain of coal, which is then raised by flights of a specially designed conveyor.
Ultimate Analysis of Coal
This analysis of coal is more precise way to find the chemical composition of coal with respect to the elements like carbon, hydrogen, oxygen, nitrogen, sulfur and ash. The content of carbon and hydrogen combines with oxygen to form carbon dioxide and water by way of combustion. The chemical analysis of coal alone is not enough to predict the suitability of coal for purpose of heating. However, the chemical composition is very useful in combustion calculations and in finding the composition of flue gases. For most purposes the proximate analysis of coal is quite sufficient. The broad range in which the constituents of coal vary by weight as determined by ultimate analysis are given below :
Coal Washing
Coal washing or coal preparation is a generic term that is used to designate the various operations performed on run of mine coal to prepare it for specific end uses, without destroying the physical identity of the coal. It is now recognized as a combination of science, art and engineering, recognized in its own right as a vital link between the production and the marketing of coal.Very dirty coals, those containing large amounts of incombustible material, could only qualify for substantial cleaning if the final selling price clearly justified it. For markets, coal cleaning would improve market potential of the coal and reduce transportation costs. Quality and cost of treated coals are highly dependent on the specific coal and the market for which it is aimed.
Coal preparation includes blending and homogenization, size reduction, grading, screening and handling and, perhaps most importantly, beneficiation or cleaning. Selection of methods and corresponding degree of beneficiation required, significantly governs the cost of coal preparation, which is greatly determined by the market suitability of the product. Therefore, there are different levels of cleaning to which a coal may be economically subjected, according to its intended utilization. Very dirty coals (those containing large amount of incombustible material), could only qualify for substantial cleaning if the final selling price justifies it.
The demand for coal preparation will grow along with increases in production for various reasons, some of which are mentioned as follows:
- Depletion of higher quality coal seams.
- Mechanized mining which increases impurities in run-of-mine coal.
- High cost of transportation, which makes it uneconomical to transport inert material.
- Market demand for higher quality coal.
- Higher mine costs, which make it imperative to improve coal washing techniques for optimized recovery.
- Environmental requirement in regard to minimizing pollution.
Coal cleaning is a process by which impurities such as sulfur, ash, and rock are removed from coal to upgrade its value. Coal cleaning processes are categorized as either physical cleaning or chemical cleaning. Physical coal cleaning processes, the mechanical separation of coal from its contaminants using differences in density, are by far the major processes in use today. Chemical coal cleaning processes are currently being developed, but their performance and cost are undetermined at this time. Therefore, chemical processes are not included in this discussion. In the initial preparation phase of coal cleaning, the raw coal is unloaded, stored, conveyed, crushed, and classified by screening into coarse and fine coal fractions. The size fractions are then conveyed to their respective cleaning processes.
The majority of coal cleaning processes use upward currents or pulses of a fluid such as water to fluidize a bed of crushed coal and impurities. The lighter coal particles rise and are removed from the top of the bed. The heavier impurities are removed from the bottom. Coal cleaned in the wet processes then must be dried in the final preparation processes.
Final preparation processes are used to remove moisture from coal, thereby reducing weight and raising the heating value. The first processing step is dewatering, in which a major portion of the water is removed by the use of screens, thickeners, and cyclones. The second step is normally thermal drying, achieved by any one of three dryer types: fluidized bed, flash, and multi-louvered. In the fluidized bed dryer, the coal is suspended and dried above a perforated plate by rising hot gases. In the flash dryer, coal is fed into a stream of hot gases for instantaneous drying. The dried coal and wet gases are both drawn up a drying column and into a cyclone for separation. In the multi-louvered dryer, hot gases are passed through a falling curtain of coal, which is then raised by flights of a specially designed conveyor.
Briquettes and their Characteristics
The term briquettes is used in respect of the dust, slack and other small size waste remains of lignite, peat, coke, etc. compressed into different shapes of regular form, with or without binder. Dust and rubble result in considerable percentage during mining, transportation, etc. and the briquetting industry is, therefore, an important step towards the saving of fuel economy. These are hard and nut shaped items used as fuel, for burning ovens chullahs in the household kitchens, small restaurants and hotels.
Good briquettes should be quite hard and as little friable as possible. They must withstand the hazards of weather, and must be suitable for storing and general handling in use. These properties are impart to briquettes by a correctly selected binder, or suitable processing such as pre-heating, pressing, etc. Amongst the binders, asphalt, pitch are most commonly used, giving fine results. Bentonite, molasses and sodium silicate are also used as bonding materials. The general conclusion is that 5-8% binder should be used to produce high quality briquettes.
Due to poverty in the rural area, the demand of coal briquettes is tremendous and is likely to increase with the increase in population of below poverty line. Beside above household demand, small restaurants, dhabas and hotels also use these coal briquettes and have similar demand to households.
The raw materials, slack coal powder, washers sink powder is mixed with molasses, bentonite and sodium silicate etc. in suitable proportions. The mixed material is then pressed into briquettes form in the power-operated machine. The briquettes so formed are sun dried. Dried briquettes are packed and sold.
Some reasons for coal storage are given below:
- Decrease of demand for coal in market,
- To be ready for the bottlenecks caused by the halts which may occur in the production,
- To meet the consumers demand without interruption,
- To produce in mild climate conditions and market it in winter,
- To decrease of moisture content of coal,
- the defects which may occur in thermal power stations and washing plants,
- To feed the thermal power stations continuously with coal of specified properties.
Besides various advantages, stacking presents also some disadvantages. Some of these are listed below:
- Stacked coal is a unprofitable investment and needs supplementary expenses.
- As a result of oxidation. coking property and calorific value of the coal are decreased,
- Oxidation of coal causes an increase in ignition temperature.
- If the coal is fragile. it will be fragmented so the percentage of small particle size material is increased.
- Oxidized coal decreases the performance of washing plants.
- As a result of storage of the coals containing high percentage of methane in closed silos which are not ventilated as required. explosive gas compositions can be formed.
Researches proved that the physical adsorption of oxygen by coal starts at -80°C. it decreases rapidly with increasing temperature and becomes insignificant after 50°C. The chemical reaction of oxygen with coal becomes important after -5°C and physical adsorption is left behind when the temperature increases over 0°C (Sevenster. 1961).
As indicated above. the reactions between oxygen and coal are exothermic. According to the findings obtained at the end of various researches. a heat of 2 to 4 calories emerges for 1 ml oxygen adsorbed to the coal under normal conditions. The heat produced as a result of reactions is generally carried by airflow and there is not any significant change in ambient temperature. However. in some cases formed heat cannot be carried away from the environment and the temperature begins to increase. The reaction gets accelerated and spread over with the increasing temperature; produced heat takes the coal to ignition temperature (around 175°C) in suitable conditions and open flamed fire begins. The time passed from the beginning of oxidation to reaching out to ignition temperature is called Incubation Period (Jones and Towned. 1949; C hakravorty. 1960).
Coal carbonization and coke oven plant
Coke and its Characteristics
It is obtained from destructive distillation of coal, being left in the shape of solid residue. Coke can be classified into two categories : soft coke and hard coke. Soft coke is obtained as the solid residue from the destructive distillation of coal in the temperature range of 600-650°C. It contains 5 to 10% volatile matter. It burns without smoke. It is extensively used as domestic fuel. Hard coke is obtained as solid residue from the destructive distillation of coal in the temperature range of 1200-1400°C. It burns with smoke and is a useful fuel for metallurgical process. Carbonization can be carried out at low temperature or high temperature. Low temperature carbonization is used to produce liquid fuels while high temperature carbonization is used to produce gaseous products.
Coal carbonization
Coal carbonization is used for processing of coal to produce coke using metallurgical grade coal. Coal carbonization involves heating of coal in the absence of air. Coke making process is multi step complex process and variety of solid liquids and gaseous products are produced which contain many valuable products. Various products from coal carbonization in addition to coke are coke oven gases. coal tar, light oil, and aqueous solution of ammonia and ammonia salt. With the development of steel industry there has been continuous development in coke oven plant since latter half of nineteenth century. to improve the process conditions, recovery of chemicals and environmental pollution control strategies and energy consumption measures.
Low temperature carbonization (450-750°C): In low temperature carbonization quantity of gaseous product is less while liquid products are large.
High temperature carbonization (above 900°C): In high temperature carbonization, the yield of gaseous product is more than liquid products with production of tar relatively low.
COKE OVEN PLANT
Due to the development of iron and steel industry coke oven plant has become an integral part of iron and steel industry. Due to increasing demand of iron and steel, there has been a considerable increase in the coke oven capacity which resulted increase output of coal chemicals.
Two types of coke manufacturing technologies use are:
- Coke making through by product recovery
- Coke making through non-recovery/ heat recovery
In India, building of coke oven batteries was initiated in the beginning of the ninth century, now about 3000 ovens are in operation/ construction in the coke oven plant. By the year 2011-12 ,the world coking coal requirement will be about 433 million metric tones in which India’s requirement is estimated to about 54 million tones. By product from coal gasification plant includes coke, coal tar, sulphur, ammonia. Coal tar distillation produces tar, benzol, cresol, phenol, creosote.
Coking Coals
Blast furnace requires coke of uniform size, high mechanical strength, and porosity with minimum volatile matter and minimum ash. Coking coal may be divided on the basis of their coking properties: prime coking coal, medium coking coal and semi coking coal. The prime coking coal produce strong metallurgical coke while coals of other groups yield hard coke, only the concentration of moisture, ash, sulphur and sometime phosphorous and ash fusion temperature are important in determining the grade of coking coal since they influence the quality of coke produced. Low moisture, ash, sulphur and phosphorous content in the coal are desirable for production of good quality coke. The desired analysis of typical coal charge to coke oven is:
Ash content : 16% ±0.5%
Moisture : 6-7%
Volatile matter : 22-25%
Fixed carbon : 58-60%%
Sulfur : 0.56%
Phosphorous : 0.09%
Some of the other factors affecting quality of coke are rank of coal, particle size, bulk density, weathering of coal, coking temperature and coking rate, soaking time and quenching practice.
Coal Handling Plant and Coal Preparation Section
Coal needs to be stored at various stages of the preparation process, and conveyed around the coal preparation section. Crushing and screening are the important part of coal handling plant. Crushing reduces the overall size of the coal so that it can be more easily processed and handled. Screens are used to ranges the size of coal. Screens can be static, or mechanically vibrated. Dewatering screens are used to remove water from the product.
Various sections in coke oven plant are
- Coal Handling Plant and Coal Preparation Section: To prepare coal blend suitable for carbonization. various steps involved are unloading and storage of coal. blending of coal of various grade, coal crushing and transport to coal storage tower.
- Partial briquetting: To prepare briquette of coal to charge along with coal into the Coke oven.
- Coke oven Batteries: To convert coal into coke by carbonizing coal in absence of air. The process steps involved are coal charging and coal carbonisation.
- Coke sorting Plant: Crushing and sorting of coke to suitable size for use in blast furnace. The steps involved are coke pushing. coke quenching. coke crushing/screening.
- Coke oven gas recovery: Collection and cleaning of coke oven gas and recovery of by products. . This involves gas cooling. tar recovery, desulfurization of coke oven gas . recovery of ammonia, recovery of light oil.
- Ammonia recovery and Ammonium Sulfate Production: Recovery of ammonia and neutralization with sulfuric acid or nitric acid in case of ammonium nitrate/calcium ammonium nitrate.
- Waste water treatment: Treatment of phenolic waste water.
Partial Briquetting
Briquettes of low grade coal are prepared using a binder (pitch/pitch+tar) up to 2 to 3.0% of charge. This partial briquette of coal is charged with coal into coke oven which significantly improve the quality of coke.
Coke oven Batteries
Coke oven plant consists of Coke oven batteries containing number of oven (around 65 ovens in each battery). The coal is charged to the coke oven through charging holes. The coal is then carbonized for 17-18 hours, during which volatile matter of coal distills out as coke oven gas and is sent to the recovery section for recovery of valuable chemicals. The ovens are maintained under positive pressure by maintaining high hydraulic main pressure of 7 mm water column in batteries. The coking is complete when the central temperature in the oven is around 950-1000°C. At this point the oven is isolated from hydraulic mains and after proper venting of residual gases, the doors are opened for coke pushing. At the end of coking period the coke mass has a high volume shrinkage which leads to detachment of mass from the walls ensuring easy pushing. The coke is then quenched and transferred to coke sorting plant. The control of oven pressure is quite important because lower pressure leads to air entry while higher pressure leads to excessive gassing, leakage of doors, stand pipe etc. Proper leveling of coal is important and care is taken so that free board space above (300 mm) is maintained to avoid choking. Metallurgical coke is produced during the carbonization of coking coal blend in a coke oven battery. This coke is produced normally in three size fractions namely coke breeze (size – 10 mm), nut coke (size +10 mm to – 25 mm) and blast furnace (BF) coke (+ 25 mm to – 80 mm). BF coke is one of the most important factors which affect the economic efficiency of a blast furnace. It also constitutes a great portion of the production costs of the hot metal. The use of nut coke in blast furnace is the essential factor to reduce the costs of iron making.
Coke oven plants are integral part of a steel plant to produce coke, which is used as fuel in the blast furnace. Coke oven plant produces important by product coal chemical tar, ammonia, crude benzol which is fractionated to produce aromatics-benzene toluene, xylene. The Coke Oven Plant consists of following sections:
Coke Oven Batteries
Coke oven are used to convert coal into coke by carbonizing coal in absence of air and there by distilling the volatile matter out of coal. Coke is taken as product which is use as fuel and as a reducing agent in smelting iron ore in a blast furnace and coke oven gas as byproduct is treated for recovery of coal chemicals. The coke oven temperature is keep as high as 2000°C.
Coke Oven Gas plant
Coke oven gas produce during the process of coking of coal are used in coke oven gas plant for the recovery of various valuable chemicals like tar, ammonia and benzol. These chemicals are recovered and gas is cleaned.
Typical Analysis of Blast Furnace Coke
- Moisture : 3.5-6 %
- Ash: 15.5-17.0%
- V.M.: <1.0%
- Sulfur: 0.65%
- Fixed Carbon: 79-81%
By-product from Coke Oven Plant:
The high temperature carbonization is used for production of coke for use in blast furnace. Various by-products obtained from coal carbonization are crude tar, crude benzol, and ammonia. Typical yield of some important byproduct are: Tar 3.2%, ammonium sulfate 1.1%, crude benzol 0.9%.
Gas Condensation section:
Coke oven gas containing water vapors and chemical products of coking (tar, ammonia, benzol etc. at temperature about 750-800°C from the coke oven plant is cooled to temperature of 80-82°C. During gas cooling 65-70% of the tar is condensed. Further cooling of gas, the water vapors and the remaining part of the tar get condensed along with some ammonia and other chemicals.
Typical Analysis of Coke Oven Gas:
Methane: 26.0%
Hydrogen: 56.5%
Hydrocarbons: 2.3%
Carbon monoxide: 8.5%
Carbon Dioxide: 3.0%
Oxygen: 0.4%
Nitrogen: 3.3%
Density: 0.0004848 g/cc
Calorific value: 4.3 cal/cc
Ammonium Sulfate Plant
The gases from exhaust goes to ESP where tar is separated and the tar free gases goes bubbled through dilute solution of sulfuric acid in saturators. Ammonia is absorbed by sulfuric acid and ammonium sulfate is formed. One tonne of coal yields about 0.3 tonne tar and 5-8 gm ammonia per cubic meter of gas.
Benzol Recovery Section
The gases from saturator goes to series of coolers and then to benzol scrubbers where benzol is scrubbed with wash oil. Benzol crude oil goes to benzol recovery section where benzol is removed and the wash oil after treatment is sent to the scrubbers. Crude Benzol thus recovered goes to benzol rectification plant. Light crude benzol contains low boiling sulfur compound, BTX, solvents, still bottom residue. Benzol after washing and neutralization with caustic soda is send to benzol column for fractionating into different fraction.
Various products of Coke Oven and Distillation of Benzol
- Coke: 76%
- Tar production: 3.3% (Pitch, PCM, anthracene oil, Naphthalene, Road tar, Cresolate, sodium phenolate, Dephenolised oil, Other oils.)
- Ammonia: 0.28% (Used for production of ammonium sulfate)
- Crude benzoyl: 0.85% (Benzene, Toluene, Xylene, Still bottom, Solvent oils)
- Coke Oven Gas: For industrial use as fuel
- Moisture & other losses: 5.04%
Coal Tar Distillation
Coal tar is produced as result of high temperature carbonization and is a viscous dark brown product with characteristic odor and consists of about 300 different products. some of the major constituents are the aromatics and heterocyclic compounds; benzene, toluene, xylene, phenol cresol, naphthalene, anthracene, phenanthrene, pyridine, carbazole, coumarone etc.
The tar distillation unit consists of:
- Distillation section
- Fractional crystallization and washing section
- Combustible mixture preparation section
- Phenol rectification section
- dolomite tar preparation unit
- Extra hard pitch preparation
Tar containing around 5% moisture is first dehydrated before distillation. The dehydrated tar is heated to 375-400°C using super heated steam to drive out the flashed vapor and the residue is taken as pitch. The oil vapor is sent to anthracene column for anthracene recovery while the vapour is sent to other column for recovery of various fraction light oil, phenol, naphthalene and heavy oil fraction. Naphthalene fraction is sent to crystalliser to separate naphthalene. Phenol is recovered from various fractions by treating with a sodium hydroxide to form sodium phenolate which is reacted with CO
2 to release phenol. Pyridine is recovered by washing different fraction with sulphuric acid.
Typical Component of Coal Tar
- Naphthalene:- 5-10%
- Phenanthrene:- 4-6%
- Carbazole:- 1-2%
- Anthracene:- 0.5-1.5%
- Phenol:- 0.2-0.5%
- Crezol:- 0.6-1.2%
- Pyridine compound:- 0.5-1.5%
Cleaner Technologies in Coke Oven Plant
Coke oven plants are one of the highly polluting industries. Continuous development has been there to reduce the pollution load and energy consumption. Some of the cleaner technology are modified wet quenching, coke dry quenching, coal moisture control,, high pressure ammonia aspiration system, modern leak proof doors, advance technologies for desulfurization of coke oven plant.

Charcoal
Charcoal is the blackish residue consisting of impure carbon obtained by removing water and other volatile constituents from animal and vegetation substances. Charcoal is usually produced by slow pyrolysis, the heating of wood, sugar, bone char, or other substances in the absence of oxygen. The resulting soft, brittle, lightweight, black, porous material resembles coal and is 85% to 98% carbon with the remainder consisting of volatile chemicals and ash.
Historically, production of wood charcoal in districts where there is an abundance of wood dates back to a very ancient period, and generally consists of piling billets of wood on their ends so as to form a conical pile, openings being left at the bottom to admit air, with a central shaft to serve as a flue. The whole pile is covered with turf or moistened clay. The firing is begun at the bottom of the flue, and gradually spreads outwards and upwards. The success of the operation depends upon the rate of the combustion. Under average conditions, 100 parts of wood yield about 60 parts by volume, or 25 parts by weight, of charcoal; small scale production on the spot often yields only about 50%, large scale was efficient to about 90% even by the 17th century.
The modern process of carbonizing wood, either in small pieces or as sawdust in cast iron retorts, is extensively practiced where wood is scarce, and also for the recovery of valuable byproducts (wood spirit, pyroligneous acid, wood tar), which the process permits. The question of the temperature of the
carbonization is important; wood becomes brown at 220 °C, a deep brown-black after some time at 280 °C, and an easily powdered mass at 310 °C. Charcoal made at 300° is brown, soft and friable, and readily inflames at 380 °C; made at higher temperatures it is hard and brittle, and does not fire until heated to
about 700 °C.
Types of charcoal
Commercial charcoal is found in either lump, briquette, or extruded forms:
- Lump charcoal is made directly from hardwood material and usually produces far less ash than briquettes.
- Briquettes are made by compressing charcoal, typically made from sawdust and other wood by-products, with a binder and other additives. The binder is usually starch. Some briquettes may also include brown coal (heat source), mineral carbon (heat source), borax, sodium nitrate (ignition aid), raw sawdust (ignition aid), and other additives like paraffin or petroleum solvents to aid in ignition.
- Extruded charcoal is made by extruding either raw ground wood or carbonized wood into logs without the use of a binder. The heat and pressure of the extruding process hold the charcoal together. If the extrusion is made from raw wood material, the extruded logs are then subsequently carbonized.
Uses
One of the most important historical applications of wood charcoal was as a constituent of gunpowder. It was also used in metallurgical operations as a reducing agent, but its application has been diminished by the introduction of coke, anthracite smalls, etc. For example, charcoal may be used to smelt a variety of metals from aluminum to copper as it burns at the necessary temperature (2000 degrees Fahrenheit/1100 degrees Celsius). A limited quantity is made up into the form of drawing crayons; but the greatest amount is used as a fuel, which burns hotter and cleaner than wood. Charcoal is often used by blacksmiths, for cooking, and for other industrial applications.
Purification/Filtration
The porosity of activated charcoal accounts for its ability to readily adsorb gases and liquids; charcoal is often used to filter water or adsorb odors. Its pharmacological action depends on the same property; it adsorbs the gases of the stomach and intestines, and also liquids and solids (hence its use in the treatment of certain poisonings). Charcoal filters are used in some types of gas mask to remove poisonous gases from inhaled air. Wood charcoal also to some extent removes coloring material from solutions, but animal charcoal is generally more effective.
Animal charcoal or bone black is the carbonaceous residue obtained by the dry distillation of bones; it contains only about 10% carbon, the remainder being calcium and magnesium phosphates (80%) and other inorganic material originally present in the bones. It is generally manufactured from the residues obtained in the glue and gelatin industries.
Environmental aspects of fuel gasification technology
Advantages:
- Easy Carbon monoxide sequestration.
- Removal of most pollutants from the gas before the turbine (solids, sulfur, heavy metals ,...)
- Low emission of SO
- NO2, and dust.
Disadvantages:
- Gasification is similar to coking of coal. .
- Hydrocarbons (benzene), hydrogen sulfide and dust are emitted.
- Effluents are produced.
Application of gasification processes:
- Power generation in integrated gasification combined cycle (IGCC) power blocks
- Production of synthesis gas for chemical technology
- Production of artificial motor fuels (synthetic petrol and diesel)
- Production of synthetic gas (SNG)
Producer Gas
Producer Gas means a fuel gas made from coke or other carbonaceous material. Air is passed over the red-hot carbonaceous fuel and carbon monoxide is produced. The reaction is exothermic and proceeds as follows:
2C + O2 → 2CO
The nitrogen in the air remains unchanged and dilutes the gas, leaving it a very low calorific value. After "scrubbing", to remove tar, the gas may be used to power gas turbines which are well-suited to fuels of low calorific value or spark ignited engines (where 100% petrol fuel replacement is possible) or diesel internal combustion engines where 40% - 15% of the original diesel fuel is still used to ignite the gas.
Producer gas is useful but requires careful handling because of the high risk of carbon monoxide poisoning.
Fuel gas for industrial use was made using producer gas technology. Producer gas is made by blowing air through an incandescent fuel bed (commonly coke or coal) in a gas producer. The reaction of fuel with insufficient air for total combustion produces carbon monoxide (CO); this reaction is exothermic and self sustaining. It was discovered that adding steam to the input air of a gas producer would increase the CV of the fuel gas by enriching it with CO and hydrogen (H2) produced by water gas reactions. Producer gas has a very low CV of 3.7 to 5.6 MJ/m³ (100-150 Btu/ft3 (std)); because the calorific gases CO/H2 are diluted with lots of inert nitrogen (from air) and carbon dioxide (CO2) (from combustion).
The problem of nitrogen dilution was overcome by the blue water gas (BWG) process, developed in the 1850s. The incandescent fuel bed would be alternately blasted with air followed by steam. The air reactions during the blow cycle are exothermic, heating up the bed, while the steam reactions during the make cycle, are endothermic and cool down the bed. The products from the air cycle contain non-calorific nitrogen and are exhausted out the stack while the products of the steam cycle are kept as blue water gas. This gas is composed almost entirely of CO and H2, and burns with a pale blue flame similar to natural gas. BWG has a CV of 11 MJ/m³ (300 Btu/ft3 (std)).
Because blue water gas lacked illuminants it would not burn with a luminous flame in a simple fishtail gas jet as existed prior to the discovery of the gas mantle in the 1890s. Various attempts were made to enrich BWG with illuminants from gas oil in the 1860s. Gas oil (an early form of gasoline) was the flammable waste product from kerosene refining, made from the lightest and most volatile fractions (tops) of crude oil.
In 1875 Thaddeus S.C. Lowe invented the Carburetted water gas process. This process revolutionized the manufactured gas industry and was the standard technology until the end of manufactured gas era. A CWG generating set consisted of three elements; a producer (generator), carburettor and a super heater connected in series with gas pipes and valves.
During a make run, steam would be passed through the generator to make blue water gas. From the generator the hot water gas would pass into the top of the carburetor where light petroleum oils would be injected into the gas stream. The light oils would be thermocracked as they came in contact with the white hot checkerwork fire bricks inside the carburettor. The hot enriched gas would then flow into the superheater, where the gas would be further cracked by more hot fire bricks.
----------------------*-*-*----------------------










































No comments:
Post a Comment