LIQUID FUELS AND THEIR CHARACTERISTICS
The liquid fuels can be classified as follows :
(a) Natural or crude oil, and
(b) Artificial or manufactured oils.
The advantages and disadvantages of liquid fuels can be summarized as follows :
Advantages
- They posses higher calorific value per unit mass than solid fuels.
- They burn without dust, ash, clinkers, etc.
- Their firing is easier and also fire can be extinguished easily by stopping liquid fuel supply.
- They are easy to transport through pipes.
- They can be stored indefinitely without any loss.
- They are clean in use and economic to handle.
- Loss of heat in chimney is very low due to greater cleanliness.
- They require less excess air for complete combustion.
- They require less furnace space for combustion.
Disadvantages
- The cost of liquid fuel is relatively much higher as compared to solid fuel.
- Costly special storage tanks are required for storing liquid fuels.
- There is a greater risk of fire hazards, particularly, in case of highly inflammable and volatile liquid fuels.
- They give bad odour.
- For efficient burning of liquid fuels, specially constructed burners and spraying apparatus are required.
Petroleum and its Characteristics
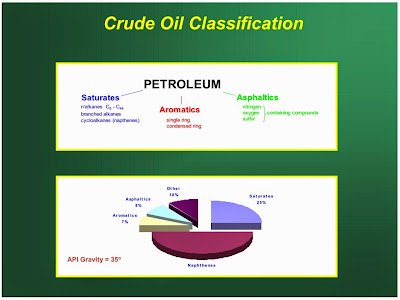
Petroleum is a basic natural fuel. It is a dark greenish brown, viscous mineral oil, found deep in earth’s crust. It is mainly composed of various hydrocarbons (like straight chain paraffins, cycloparaffins or napthenes, olefins, and aromatics) together with small amount of organic compounds containing oxygen nitrogen and sulphur. The average composition of crude petroleum is : C = 79.5 to 87.1%; H = 11.5 to 14.8%; S = 0.1 to 3.5%, N and O = 0.1 to 0.5%.
Petroleums are graded according to the following physico-chemical properties :
- Specific gravity,
- Calorific value,
- Flash point and ignition point,
- Viscosity,
- Sulfur content,
- Moisture and sediment content, and
- Specific heat and coefficient of expansion.
Crude oils vary widely in appearance and viscosity from field to field. They range in colour, odour, and in the properties they contain. While all crude oils are essentially hydrocarbons, the differences in properties, specially the variations in molecular structure, mean that a crude is more or less easy to produce, pipeline, and refine. The variations may even influence its suitability for certain products and the quality of those products.
Classification of Petroleum
The chemical nature of crude petroleum varies with the part of the world in which it is found. Crudes are roughly classified into three groups, according to the nature of the hydrocarbons they contain.
Paraffinic Base Type Crude Petroleum
This type of petroleum is mainly composed of the saturated hydrocarbons from CH4 to C35 H72 and a little of the napthenes and aromatics. The hydrocarbons from C18 H38 to C35 H72 are sometimes called waxes. These contain higher molecular weight paraffins which are solid at room temperature, but little or no asphaltic (bituminous) matter. They can produce high-grade lubricating oils.
Asphaltic Base Type Crude Petroleum
It contains mainly cycloparaffins or napthenes and large proportions of asphaltic matter with smaller amount of paraffins and aromatic hydrocarbons. Some are predominantly naphthenes so yield a lubricating oil that is more sensitive to temperature changes than the paraffin-base crudes.
Mixed Base Type Crude Petroleum
The "gray area" between the two types above. Both paraffins and naphthenes/ asphaltic hydrocarbons are present, as well as aromatic hydrocarbons, and are generally rich in semi-solid waxes. . Most crudes fit this category.
BASICS OF CRUDE OIL
Crude oils are complex mixtures containing many different hydrocarbon compounds that vary in appearance and composition from one oil field to another. Crude oils range in consistency from water to tar-like solids, and in color from clear to black. An "average" crude oil contains about 84% carbon, 14% hydrogen, 1%-3% sulfur, and less than 1% each of nitrogen, oxygen, metals, and salts. Crude oils are generally classified as paraffinic, naphthenic, or aromatic, based on the predominant proportion of similar hydrocarbon molecules. Mixed-base crudes have varying amounts of each type of hydrocarbon. Refinery crude base stocks usually consist of mixtures of two or more different crude oils.
Crude oils are also defined in terms of API (American Petroleum Institute) gravity. The higher the API gravity, the lighter is the crude. For example, light crude oils have high API gravities and low specific gravities. Crude oils with low carbon, high hydrogen, and high API gravity are usually rich in paraffins and tend to yield greater proportions of gasoline and light petroleum products; those with high carbon, low hydrogen, and low API gravities are usually rich in aromatics.
API gravity (degrees) = (141.5/sp gr 60/60°F) – 131.5
Crude oils that contain appreciable quantities of hydrogen sulfide or other reactive sulfur compounds are called "sour." Those with less sulfur are called "sweet." Some exceptions to this rule are West Texas crudes, which are always considered "sour" regardless of their H2S content, and Arabian high-sulfur crudes, which are not considered "sour" because their sulfur compounds are not highly reactive.
BASICS OF HYDROCARBON CHEMISTRY
Crude oil is a mixture of hydrocarbon molecules, which are organic compounds of carbon and hydrogen atoms that may include from one to 60 carbon atoms. The properties of hydrocarbons depend on the number and arrangement of the carbon and hydrogen atoms in the molecules. The simplest hydrocarbon molecule is one carbon atom linked with four hydrogen atoms: methane. All other variations of petroleum hydrocarbons evolve from this molecule.
Hydrocarbons containing up to four carbon atoms are usually gases, those with 5 to 19 carbon atoms are usually liquids, and those with 20 or more are solids. The refining process uses chemicals, catalysts, heat, and pressure to separate and combine the basic types of hydrocarbon molecules naturally found in crude oil into groups of similar molecules. The refining process also rearranges their structures and bonding patterns into different hydrocarbon molecules and compounds. Therefore it is the type of hydrocarbon (paraffinic, naphthenic, or aromatic) rather than its specific chemical compounds that is significant in the refining process.
Three Principal Groups or Series of Hydrocarbon Compounds that occur naturally in Crude Oil.
b. Aromatics are unsaturated ring-type (cyclic) compounds which react readily because they have carbon atoms that are deficient in hydrogen. All aromatics have at least one benzene ring (a single-ring compound characterized by three double bonds alternating with three single bonds between six carbon atoms) as part of their molecular structure. Naphthalenes are fused double-ring aromatic compounds. The most complex aromatics, polynuclears (three or more fused aromatic rings), are found in heavier fractions of crude oil.
c. Naphthenes are saturated hydrocarbon groupings with the general formula CnH2n, arranged in the form of closed rings (cyclic) and found in all fractions of crude oil except the very lightest. Single-ring naphthenes (monocycloparaffins) with five and six carbon atoms predominate, with two-ring naphthenes (dicycloparaffins) found in the heavier ends of naphtha.
Other Hydrocarbons
a. Alkenes are mono-olefins with the general formula CnH2n and contain only one carbon-carbon double bond in the chain. The simplest alkene is ethylene, with two carbon atoms joined by a double bond and four hydrogen atoms. Olefins are usually formed by thermal and catalytic cracking and rarely occur naturally in unprocessed crude oil.
b. Dienes and Alkynes. Dienes, also known as diolefins, have two carbon-carbon double bonds. The alkynes, another class of unsaturated hydrocarbons, have a carbon-carbon triple bond within the molecule. Both these series of hydrocarbons have the general formula CnH2n-2. Diolefins such as 1,2-butadiene and 1,3-butadiene, and alkynes such as acetylene, occur in C5 and lighter fractions from cracking. The olefins, diolefins, and alkynes are said to be unsaturated because they contain less than the amount of hydrogen necessary to saturate all the valences of the carbon atoms. These compounds are more reactive than paraffins or naphthenes and readily combine with other elements such as hydrogen, chlorine, and bromine.
Nonhydrocarbons
a. Sulfur Compounds. Sulfur may be present in crude oil as hydrogen sulfide (H2S), as compounds (e.g. mercaptans, sulfides, disulfides, thiophenes, etc.) or as elemental sulfur. Each crude oil has different amounts and types of sulfur compounds, but as a rule the proportion, stability, and complexity of the compounds are greater in heavier crude-oil fractions. Hydrogen sulfide is a primary contributor to corrosion in refinery processing units. Other corrosive substances are elemental sulfur and mercaptans. Moreover, the corrosive sulfur compounds have an obnoxious odor.
Pyrophoric iron sulfide results from the corrosive action of sulfur compounds on the iron and steel used in refinery process equipment, piping, and tanks. The combustion of petroleum products containing sulfur compounds produces undesirables such as sulfuric acid and sulfur dioxide. Catalytic hydrotreating processes such as hydrodesulfurization remove sulfur compounds from refinery product streams. Sweetening processes either remove the obnoxious sulfur compounds or convert them to odorless disulfides, as in the case of mercaptans.
b. Oxygen Compounds. Oxygen compounds such as phenols, ketones, and carboxylic acids occur in crude oils in varying amounts.
c. Nitrogen Compounds. Nitrogen is found in lighter fractions of crude oil as basic compounds, and more often in heavier fractions of crude oil as nonbasic compounds that may also include trace metals such as copper, vanadium, and/or nickel. Nitrogen oxides can form in process furnaces. The decomposition of nitrogen compounds in catalytic cracking and hydrocracking processes forms ammonia and cyanides that can cause corrosion.
d. Trace Metals. Metals, including nickel, iron, and vanadium are often found in crude oils in small quantities and are removed during the refining process. Burning heavy fuel oils in refinery furnaces and boilers can leave deposits of vanadium oxide and nickel oxide in furnace boxes, ducts, and tubes. It is also desirable to remove trace amounts of
arsenic, vanadium, and nickel prior to processing as they can poison certain catalysts.
e. Salts. Crude oils often contain inorganic salts such as sodium chloride, magnesium chloride, and calcium chloride in suspension or dissolved in entrained water (brine). These salts must be removed or neutralized before processing to prevent catalyst poisoning, equipment corrosion, and fouling. Salt corrosion is caused by the hydrolysis of some metal chlorides to hydrogen chloride (HCl) and the subsequent formation of hydrochloric acid when crude is heated. Hydrogen chloride may also combine with ammonia to form ammonium chloride (NH4Cl), which causes fouling and corrosion.
f. Carbon Dioxide. Carbon dioxide may result from the decomposition of bicarbonates present in or added to crude, or from steam used in the distillation process.
g. Naphthenic Acids. Some crude oils contain naphthenic (organic) acids, which may become corrosive at temperatures above 450° F when the acid value of the crude is above a certain level.
Gasoline or petrol is a liquid mixture primarily used as fuel in internal combustion engines. It is petroleum-derived, and consists mostly of aliphatic hydrocarbons, enhanced with iso-octane or the aromatic hydrocarbons toluene and benzene to increase its octane rating.
Gasoline is a mixture of hydrocarbons, although some may contain significant quantities of ethanol and some may contain small quantities of additives such as methyl tert-butyl ether as anti-knock agents to increase the octane rating or as an oxygenate to reduce emissions. The hydrocarbons consist of a mixture of n-paraffins, naphthenes, olefins and aromatics. Naphthenes, olefins and aromatics increase the octane rating of the gasoline whereas the n-paraffins have the opposite effect.
Gasoline is produced in oil refineries. Material that is separated from crude oil via distillation, called virgin or straightrun gasoline, does not meet the required specifications for modern engines (in particular octane rating, but will form part of the blend. The bulk of a typical gasoline consists of hydrocarbons with between 5 and 12 carbon atoms per molecule.
Many of these hydrocarbons are considered hazardous substances and are regulated in the United States by Occupational Safety and Health Administration. The Material Safety Data Sheet for unleaded gasoline shows at least fifteen hazardous chemicals occurring in various amounts. These include benzene (up to 5% by volume), toluene (up to 35% by volume), naphthalene (up to 1% by volume), trimethylbenzene (up to 7% by volume), MTBE (up to 18% by volume) and about ten others.
The various refinery streams blended together to make gasoline all have different characteristics. Some important streams are:
- Reformate, produced in a catalytic reformer with a high octane rating and high aromatic content, and very low olefins (alkenes).
- Cat Cracked Gasoline or Cat Cracked Naphtha, produced from a catalytic cracker, with a moderate octane rating, high olefins (alkene) content, and moderate aromatics level. Here, "cat" is short for "catalytic".
- Hydrocrackate (Heavy, Mid, and Light), produced from a hydrocracker, with medium to low octane rating and moderate aromatic levels.
- Virgin or Straight-run Naphtha (has many names), directly from crude oil with low octane rating, low aromatics (depending on the crude oil), some naphthenes (cycloalkanes) and no olefins (alkenes).
- Alkylate, produced in an alkylation unit, with a high octane rating and which is pure paraffin (alkane), mainly branched chains.
- Isomerate (various names) which is obtained by isomerising the pentane and hexane in light virgin naphthas to yield their higher octane isomers.
Overall a typical gasoline is predominantly a mixture of paraffins (alkanes), naphthenes (cycloalkanes), and olefins (alkenes). Currently many countries set tight limits on gasoline aromatics in general, benzene in particular, and olefin (alkene) content. This is increasing the demand for high octane pure paraffin (alkane) components, such as alkylate, and is forcing refineries to add processing units to reduce the benzene content.
Gasoline can also contain some other organic compounds: such as organic ethers (deliberately added), plus small levels of contaminants, in particular sulfur compounds such as disulfides and thiophenes. Some contaminants, in particular thiols and hydrogen sulfide, must be removed because they cause corrosion in engines. Sulfur compounds are usually removed by hydrotreating, yielding hydrogen sulfide which can then be transformed into elemental sulfur via the Claus process.
The density of gasoline is 0.71–0.77 kg/l (0.71–0.77 g/cm3), (in English units, approx. 0.026 lb/cu in or 6.073 lb/U.S. gal or 7.29 lb/imp gal) which means it floats on water. This may be advantageous in the event of a spill. It is flammable and can burn while floating over water.
Gasoline is more volatile than diesel oil, Jet-A or kerosene, not only because of the base constituents, but because of the additives that are put into it. The final control of volatility is often achieved by blending with butane. The Reid Vapor Pressure (RVP) test is used to measure the volatility of gasoline. The desired volatility depends on the ambient temperature: in hotter climates, gasoline components of higher molecular weight and thus lower volatility are used. In cold climates, too little volatility results in cars failing to start. In hot climates, excessive volatility results in what is known as "vapour lock" where combustion fails to occur, because the liquid fuel has changed to a gaseous fuel in the fuel lines, rendering the fuel pump ineffective and starving the engine of fuel.
An important characteristic of gasoline is its octane rating, which is a measure of how resistant gasoline is to the abnormal combustion phenomenon known as pre-detonation (also known as knocking, pinging, spark knock, and other names). Octane rating is measured relative to a mixture of 2,2,4-trimethylpentane (an isomer of octane) and n-heptane. There are a number of different conventions for expressing the octane rating; therefore, the same fuel may be labeled with a different number, depending upon the system used.
A high octane fuel such as Liquefied petroleum gas (LPG) has a lower energy content than lower octane gasoline, resulting in an overall lower power output at the regular compression ratio an engine ran at on gasoline. However, with an engine tuned to the use of LPG (i.e. via higher compression ratios such as 12:1 instead of 8:1), this lower power output can be overcome. This is because higher-octane fuels allow for a higher compression ratio - this means less space in a cylinder on its combustion stroke, hence a higher cylinder temperature which improves efficiency according to Carnot's theorem, along with fewer wasted hydrocarbons (therefore less pollution and wasted energy), bringing higher power levels coupled with less pollution overall because of the greater efficiency.
The main reason for the lower energy content (per litre) of LPG in comparison to gasoline is that it has a lower density. Energy content per kilogram is higher than for gasoline (higher hydrogen to carbon ratio). The weight-density of gasoline is about 740 kg/m³ (6.175 lb/US gal; 7.416 lb/imp gal).
Oxygenate blending
Oxygenate blending adds oxygen to the fuel in oxygen-bearing compounds such as MTBE, ETBE and ethanol, and so reduces the amount of carbon monoxide and unburned fuel in the exhaust gas, thus reducing smog. In many areas throughout the US oxygenate blending is mandated by EPA regulations to reduce smog and other airborne polutants. For example, in Southern California, fuel must contain 2% oxygen by weight, resulting in a mixture of 5.6% ethanol in gasoline. The resulting fuel is often known as reformulated gasoline (RFG) or oxygenated gasoline.
Kerosene
Kerosene is a thin, clear liquid formed from hydrocarbons, with density of 0.78-0.81g/cm3. Kerosene is obtained from the fractional distillation of petroleum between 150 °C and 275 °C, resulting in a mixture of carbon chains containing 12 to 15 carbon atoms. As a heating fuel, it is often used in portable stoves, and is sold in some filling stations. It is sometimes used as a heat source during power failures. The use of portable kerosene heaters is not recommended for closed indoor areas without a chimney due to the danger of build-up of carbon monoxide gas.
A notable exception, discovered in the early 19th century, is the use of a mantle above the wick on a kerosene lamp. Looking like a delicate woven bag above the woven cotton wick, the mantle was a residue of mineral material (thorium dioxide) which glowed white hot as it burned the volatile gases emanating from the blue flame at the base of the wick. These types of lamps are still in use today in areas of the world without electricity.
Today kerosene is mainly used in fuel for jet engines. One form of the fuel is burned with liquid oxygen as rocket fuel. These fuel grade kerosenes meet specifications for smoke points and freeze points.
In countries such as India and Japan, kerosene is the main fuel used for cooking, especially by the poor. Kerosene stoves have replaced traditional wood-based cooking appliances. The price of kerosene can be a major political and environmental issue; the Indian government subsidizes the fuel to keep the price very low. Lower prices discourage dismantling of forests for cooking fuel.
Jet fuel is a type of aviation fuel designed for use in aircraft powered by gas-turbine engines. At some airports, underground fuel pipes allow refuelling without the need for tank trucks. Trucks just carry the necessary hoses and pressure apparatus, but no fuel.
Aviation fuel is a specialized type of petroleum-based fuel used to power aircraft. It is generally of a higher quality than fuels used in less critical applications such as heating or road transport, and often contains additives to reduce the risk of icing or explosion due to high temperatures, amongst other properties.
Jet fuel is a clear to straw colored fuel, based on either an unleaded paraffin oil (Jet A-1), or a naphtha-kerosene blend (Jet B). It is similar to diesel fuel, and can be used in either compression ignition engines or turbine engines. Aviation fuels consist of blends of over a thousand chemicals, primarily Hydrocarbons (paraffins, olefins, naphthenes, and aromatics) as well as additives such as antioxidants and metal deactivators, and impurities. Principal components include n-octane and isooctane. Like other fuels, blends of Aviation fuel used in piston engined aircraft are often described by their Octane rating.
Safety precautions
Any fuelling operation can be very dangerous, and aviation fuelling has a number of unique characteristics which must be accommodated. As an aircraft flies through the air, it can accumulate a charge of static electricity. If this is not dissipated before fuelling, an electric arc can occur which may ignite fuel vapours. To prevent this, aircraft are electrically bonded to the fuelling apparatus before fuelling begins, and are not disconnected until fuelling is complete. Some regions require that the aircraft and/or fuel truck be grounded as well.
Aviation fuel can cause severe environmental damage, and all fuelling vehicles must carry equipment to control fuel spills. In addition, fire extinguishers must be present at any fuelling operation, and airport firefighting forces are specially trained and equipped to handle aviation fuel fires and spills. Aviation fuel must be checked daily and before every flight for contaminants such as water or dirt.
Diesel
The word "diesel" is derived from the German inventor Rudolf Christian Karl Diesel who in 1892 invented the diesel engine. Diesel engines are a type of internal combustion engine. Rudolf Diesel originally designed the diesel engine to use coal dust as a fuel. He also experimented with various oils, including some vegetable oils, such as peanut oil, which was used to power the engines which he exhibited at the 1900 Paris Exposition and the 1911 World's Fair in Paris.
Petroleum diesel, also called Petrodiesel, or fossil diesel is produced from petroleum and is a hydrocarbon mixture, obtained in the fractional distillation of crude oil between 200 °C and 350 °C at atmospheric pressure.
Unlike Petroleum ether and Liquefied petroleum gas engines, diesel engines do not use high voltage spark ignition (spark plugs). An engine running on diesel compresses the air inside the cylinder to high pressures and temperatures (compression ratios from 15:1 to 25:1 are common); the diesel is generally injected directly into the cylinder near the end of the compression stroke. The high temperatures inside the cylinder causes the diesel fuel to react with the oxygen in the mix (burn or oxidize), heating and expanding the burning mixture in order to convert the thermal/pressure difference into mechanical work; i.e., to move the piston. (Glow plugs are used to assist starting the engine to preheat cylinders to reach a minimum operating temperature.) The high compression ratios and throttleless operation of the diesels generally result in diesel engines being more efficient than many spark-ignited engines.
This and being less flammable and explosive than gasoline are the main reasons for military use of diesel in armoured fighting vehicles like tanks and trucks. Engines running on diesel also provide more torque and are less likely to stall; as they run at a lower RPM, they will rather stutter a lot before stalling.
A disadvantage of diesel as a vehicle fuel in some climates, compared to gasoline or other petroleum derived fuels, is that its viscosity increases quickly as the fuel's temperature decreases, turning into a non-flowing gel at temperatures as high as -19 °C (-2.2 °F) or -15 °C (+5 °F), which can't be pumped by regular fuel pumps. Special low temperature diesel contains additives that keep it in a more liquid state at lower temperatures, yet starting a diesel engine in very cold weather still poses considerable difficulties.
Petroleum-derived diesel is composed of about 75% saturated hydrocarbons (primarily paraffins including n, iso, and cycloparaffins), and 25% aromatic hydrocarbons (including naphthalenes and alkylbenzenes). The average chemical formula for common diesel fuel is C 12H23, ranging from approx. C10H20 to C15H28.
Wood, hemp, straw, corn, garbage, food scraps, and sewage-sludge may be dried and gasified to synthesis gas. After purification the Fischer-Tropsch process is used to produce synthetic diesel. This means that synthetic diesel oil may be one route to biomass based diesel oil. Such processes are often called biomass-to-liquids or BTL.
Synthetic diesel may also be produced out of natural gas in the gas-to-liquid (GTL) process or out of coal in the coalto-liquid (CTL) process. Such synthetic diesel has 30% less particulate emissions than conventional diesel.
Diesel combustion exhaust is a major source of atmospheric soot and fine particles, which is a fraction of air pollution implicated in human heart and lung damage. Diesel exhaust also contains nanoparticles which have been found to damage the cardiovascular system in a mouse model. The study of nanotoxicology is still in its infancy, and the extent of health and societal effects caused by diesel combustion is unknown. Biodiesel and biodiesel blends result in greatly decreased pollution levels.
Furnace Oil (FO)
Furnace oil is a Dark, viscous residual fuel oil which is obtained by blending residual products from various refining processes with suitable diluent usually middle distillates to obtain the required fuel oil grades. These fuel oil grades are similar in nature and have been marketed under different specifications in various countries. In India it is sold under BIS specification under IS 1593-1982, Medium Grade 2 .
Uses of Furnace Oil are :
- As fuel for Power Generation in DG Sets
- As fuel for Boilers/ Furnaces/ Air preheater/ Any other Heaters
- Fuel for Bunkering
- Fuel/ Feedstock in Fertilizer Plants
Furnace oil is a class C product having Flash Point above 66°C. Since this is a residual fuel, there has to be gradual filtration system to prevent the filter choking and fuel nozzles choking. Due to its viscous nature, it has to be heated to improve its flowability and to a proper temperature for proper atomisation. Normally gear pumps are preferred to avoid cavitation problems.
Residual fuel oils are sometimes called light when they have been mixed with distillate fuel oil, while distillate fuel oils are called heavy when they have been mixed with residual fuel oil. Heavy gas oil, for example, is a distillate that contains residual fuel oil. The ready availability of very heavy grades of fuel oil is often due to the success of catalytic cracking of fuel to release more valuable fractions and leave heavy residue.
Bunker fuel is technically any type of fuel oil used aboard ships. It gets its name from the containers on ships and in ports that it is stored in; in the days of steam they were coal bunkers but now they are bunker-fuel tanks.
Residual fuel oil is less useful because it is so viscous that it has to be heated with a special heating system before use and it contains relatively high amounts of pollutants, particularly sulfur, which forms sulfur dioxide upon combustion. However, its undesirable properties make it very cheap. In fact, it is the cheapest liquid fuel available. Since it requires heating before use, residual fuel oil cannot be used in road vehicles, boats or small ships, as the heating equipment takes up valuable space and makes the vehicle heavier. Heating the oil is also a delicate procedure, which is inappropriate to do on small, fast moving vehicles. However, power plants and large ships are able to use residual fuel oil.
Heavy fuel oils continue to be used in the boiler "lighting up" facility in every coal-fired power plant. Although on an enormous scale, this use is analogous to lighting kindling to start a fire; without performing this simple function it is difficult to begin the large-scale combustion process.
-----------------------*-*-*-----------------------